Science
US Researchers Create Fertilizable Eggs from Skin Cells
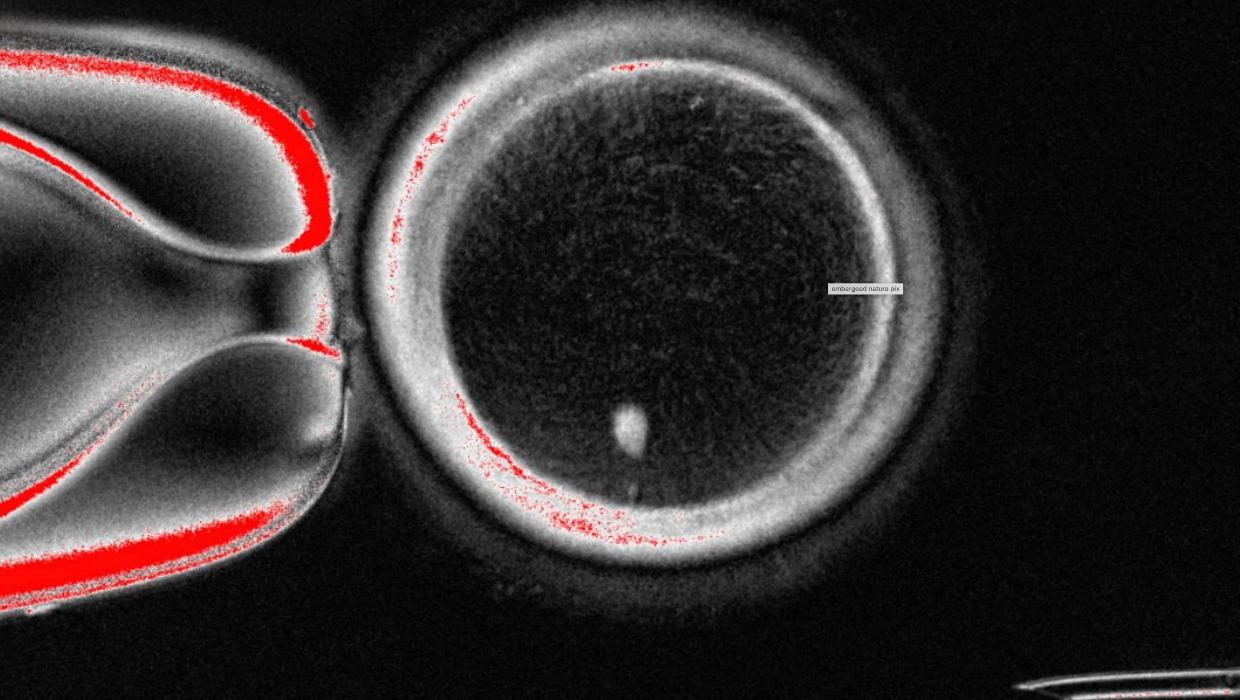
A team of scientists from the United States has successfully transformed human skin cells into fertilizable eggs, marking a significant milestone in reproductive science. This groundbreaking research, conducted at the University of California, Los Angeles (UCLA), offers new possibilities for developing lab-grown eggs and sperm, potentially aiding individuals facing fertility challenges.
The findings, published in the prestigious journal Nature, detail how researchers utilized a process called cellular reprogramming. By manipulating skin cells, they were able to create oocytes, which are immature eggs that can be fertilized. This innovative approach not only demonstrates the potential for creating gametes from non-reproductive tissue but also raises questions about the future of reproductive technologies.
Implications for Fertility Treatments
The implications of this research could be profound. For many couples struggling with infertility, options are often limited, relying heavily on donor eggs or sperm. The ability to generate viable eggs and sperm from skin cells could provide new avenues for those who cannot conceive through traditional methods.
Dr. Katherine L. E. Johnson, the lead researcher on the project, emphasized the importance of this work. “This is a critical step towards finding solutions for infertility,” she said. “Our goal is to create gametes that not only function well but also have the potential to lead to successful pregnancies.”
While the research is still in its early stages, the scientists aim to refine their techniques and understand the full viability of the lab-grown cells. The team is also exploring the ethical implications of their work, as the ability to produce gametes from non-reproductive tissues raises significant moral and social considerations.
Future Directions and Ethical Considerations
As this field of research progresses, scientists are aware of the ethical complexities surrounding the creation of human reproductive cells. Discussions about the potential for genetic modifications, the implications of designer babies, and access to such technologies are already underway.
Funding for this research comes from a combination of governmental and private sources, reflecting the growing interest in advancing reproductive technologies. The potential market for lab-grown gametes could be substantial, with fertility treatments already representing a multi-billion dollar industry.
Researchers are optimistic about the future of their findings, as they seek to overcome the current limitations of infertility treatments. The success of this study at UCLA could pave the way for further advancements, making it possible for individuals and couples worldwide to conceive when they might not have been able to otherwise.
As the scientists continue their work, the global community will be watching closely. The potential for lab-grown eggs and sperm could redefine the landscape of reproductive health, offering hope to many who wish to start families.
-

 World3 months ago
World3 months agoTest Your Knowledge: Take the Herald’s Afternoon Quiz Today
-

 Sports3 months ago
Sports3 months agoPM Faces Backlash from Fans During Netball Trophy Ceremony
-

 Lifestyle3 months ago
Lifestyle3 months agoDunedin Designers Win Top Award at Hokonui Fashion Event
-

 Sports3 months ago
Sports3 months agoLiam Lawson Launches New Era for Racing Bulls with Strong Start
-

 Lifestyle3 months ago
Lifestyle3 months agoDisney Fan Reveals Dress Code Tips for Park Visitors
-

 Health3 months ago
Health3 months agoWalking Faster Offers Major Health Benefits for Older Adults
-

 World3 months ago
World3 months agoCoalition Forms to Preserve Māori Wards in Hawke’s Bay
-

 Politics3 months ago
Politics3 months agoScots Rally with Humor and Music to Protest Trump’s Visit
-

 Top Stories3 months ago
Top Stories3 months agoUK and India Finalize Trade Deal to Boost Economic Ties
-

 World3 months ago
World3 months agoHuntly Begins Water Pipe Flushing to Resolve Brown Water Issue
-

 Entertainment3 months ago
Entertainment3 months agoExperience the Excitement of ‘Chief of War’ in Oʻahu
-

 Science3 months ago
Science3 months agoNew Interactive Map Reveals Wairarapa Valley’s Geological Secrets









